Background / Methods
Propofol Alpha and Slow Wave
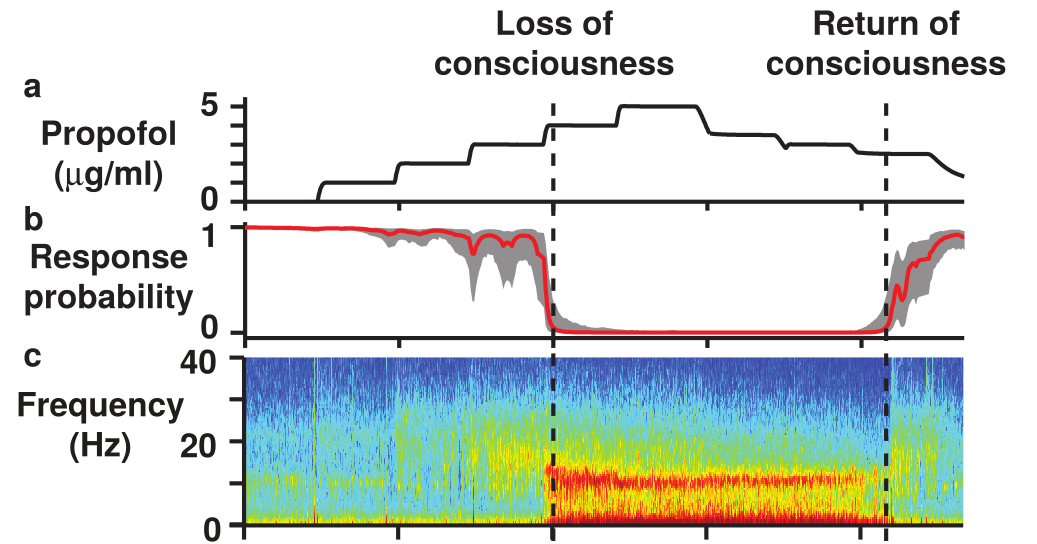 { width=100%
}
{ width=100%
}
Why do we care?
- Better understanding of propofol mechanisms could lead to more targeted
anesthetics
- Clarify mechanistic differences between between anesthesia and sleep,
including rhythms
- Propofol coupling correlates with depth of anesthesia, as can be used in the
Operating Room
- Coupling mechanisms may tie to specific aspects of loss of consciousness
Sleep Spindles vs Propofol Alpha
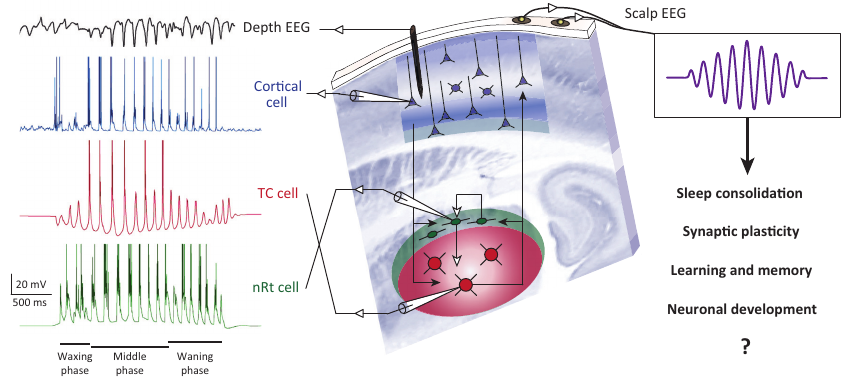 {
width=100% }
{
width=100% }
Propofol Mechanisms of Action
- Increases $GABA_A$ inhibition:
- Increases max synaptic conductance ($\uparrow\bar g_{GABA_A}$ )
- Increases decay time constant ($\uparrow tau_{GABA_A}$ )
- Decreases thalamocortical (TC) cell H-current conductance ($\downarrow \bar g_H$ )
- Decreases Excitation from brainstem ($\downarrow I_{applied}$)
Our Model Thalamus
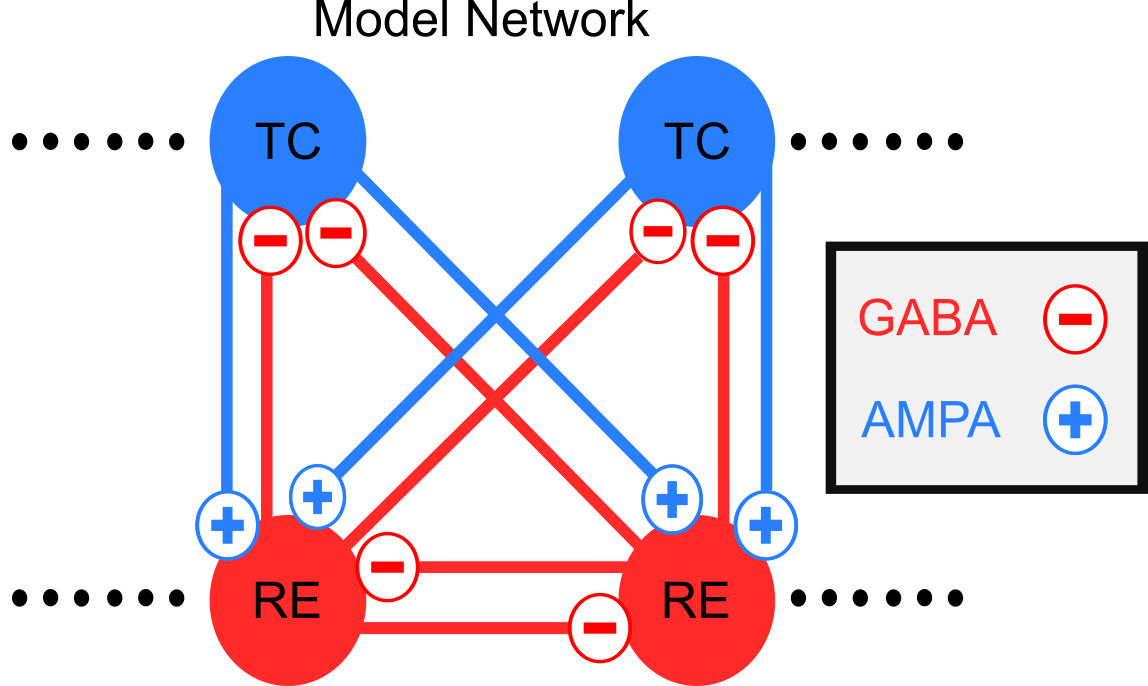 { width=80% }
{ width=80% }
Overview
Overview
- Increase of $GABA_A$ and decrease of TC cell H-current are required for
thalamic Alpha oscillations
- Thalamic Alpha oscillations are sustained spindles
- Interaction between thalamic Alpha and Slow Wave Activity can produce
propofol phase-amplitude coupling regimes
$GABA_A$ and H-current changes are required for thalamic Alpha oscillations
Native hyperpolarized thalamus cannot produce Alpha oscillations
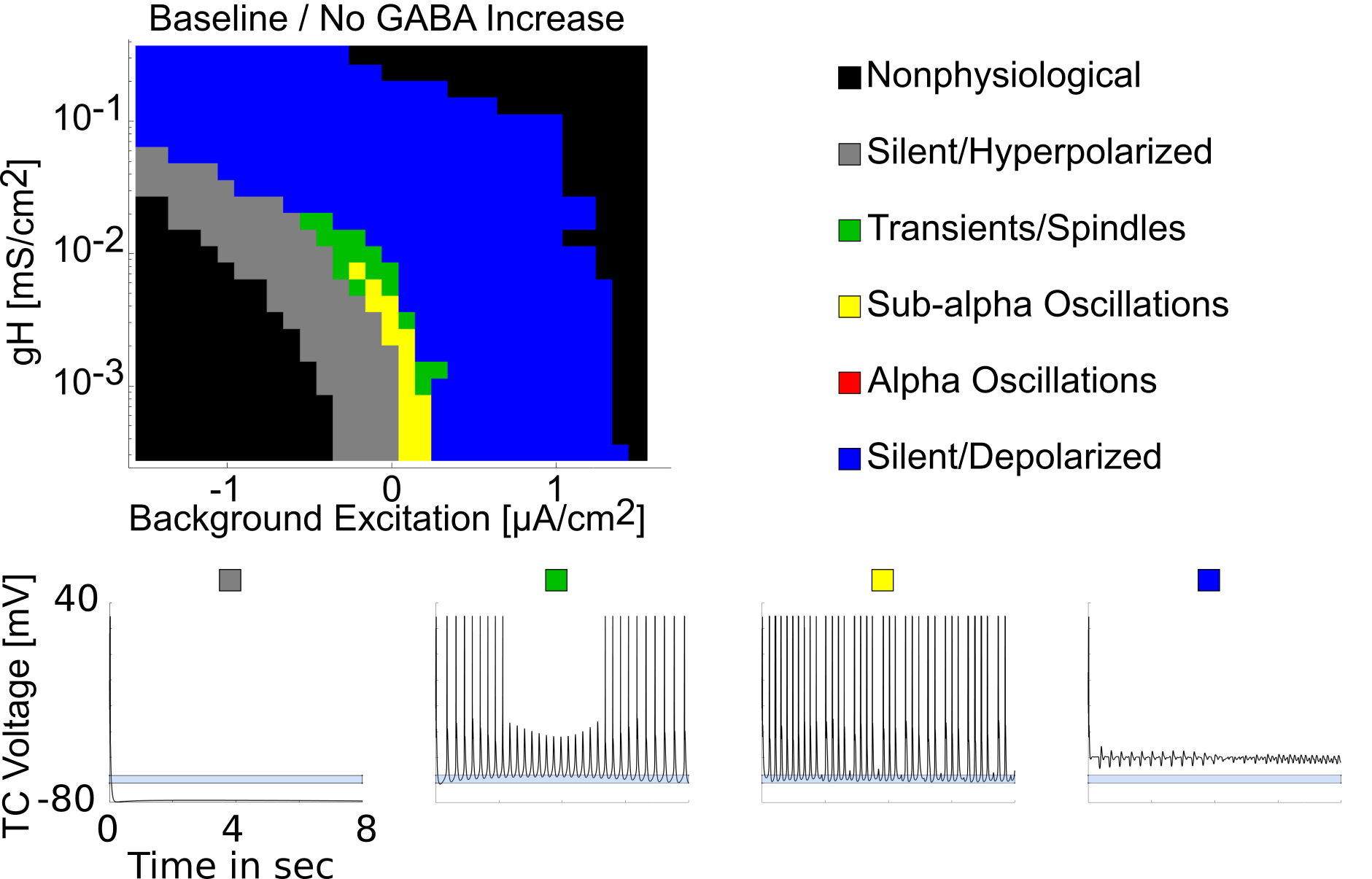 { width=75% }
{ width=75% }
Simulating $GABA_A$ increase enables thalamic Alpha oscillations
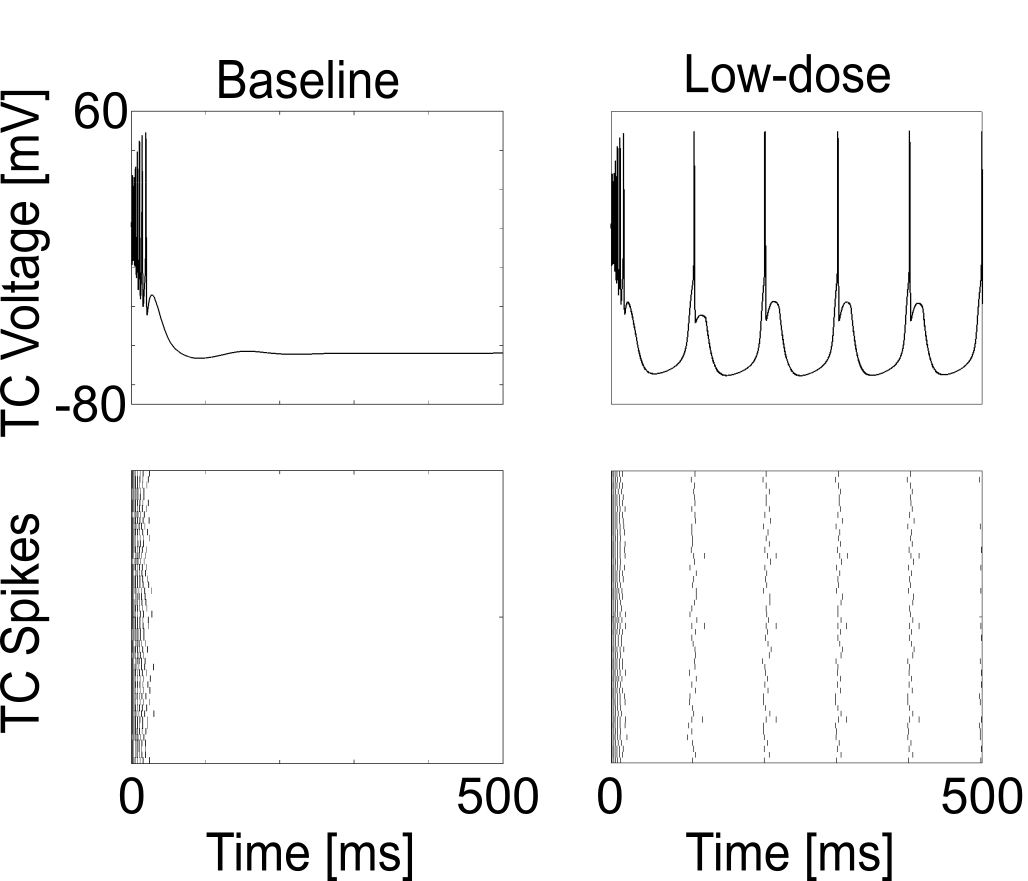 { width=55% }
{ width=55% }
Alpha requires H-current decrease
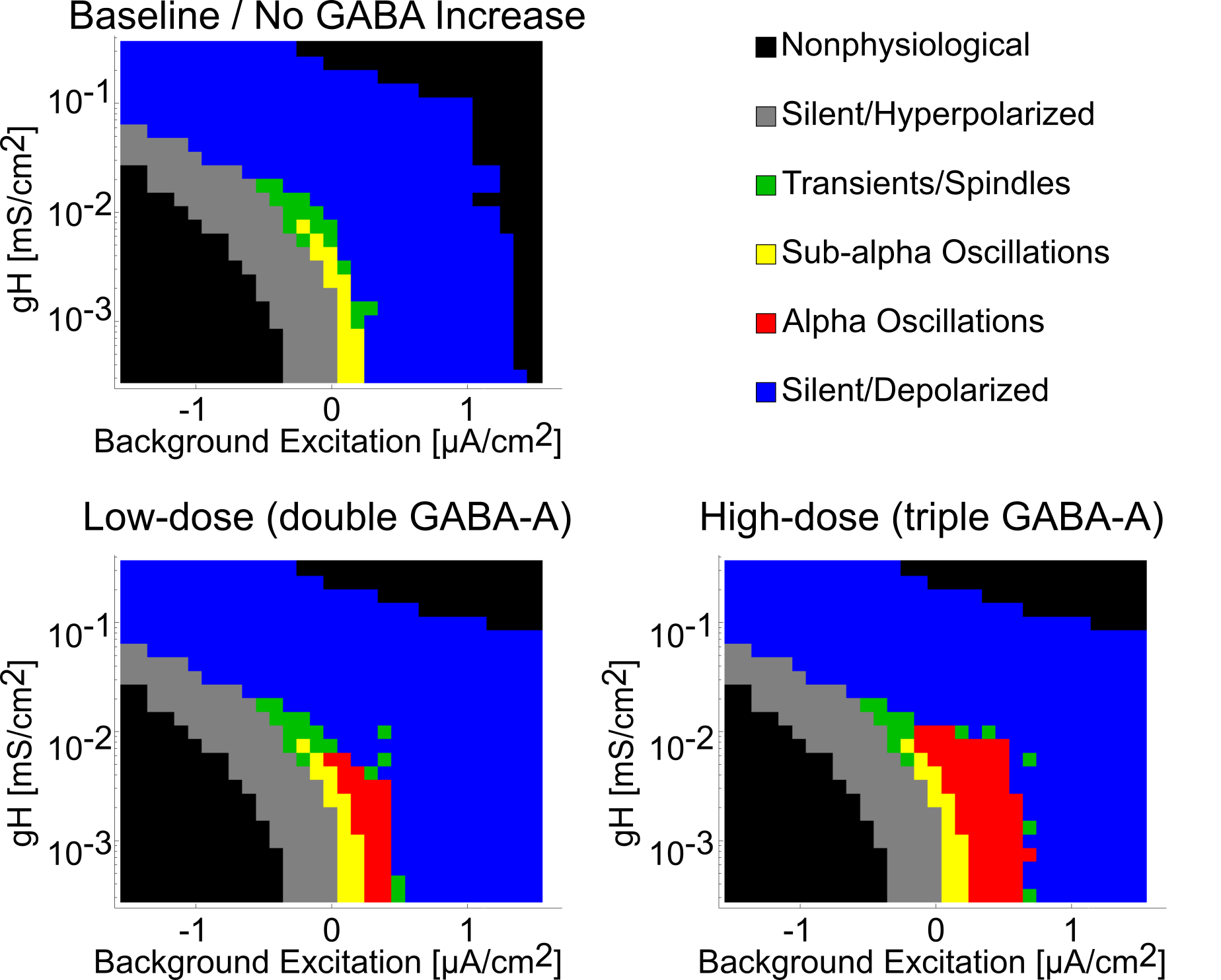 {
width=65% }
{
width=65% }
Summary So Far
- Sustained Alpha does not occur normally
- $GABA_A$ increase is a necessary factor for sustained Alpha
- TC cell H-current decrease is also a necessary factor for sustained Alpha
Overview So Far
- Increase of $GABA_A$ and decrease of TC cell H-current are required for
thalamic Alpha oscillations
- Thalamic Alpha oscillations are sustained spindles
- Interaction between thalamic Alpha and Slow Wave Activity can produce
propofol phase-amplitude coupling regimes
Thalamic Alpha oscillations are sustained spindles
Sustained alpha emerges from Baseline spindles
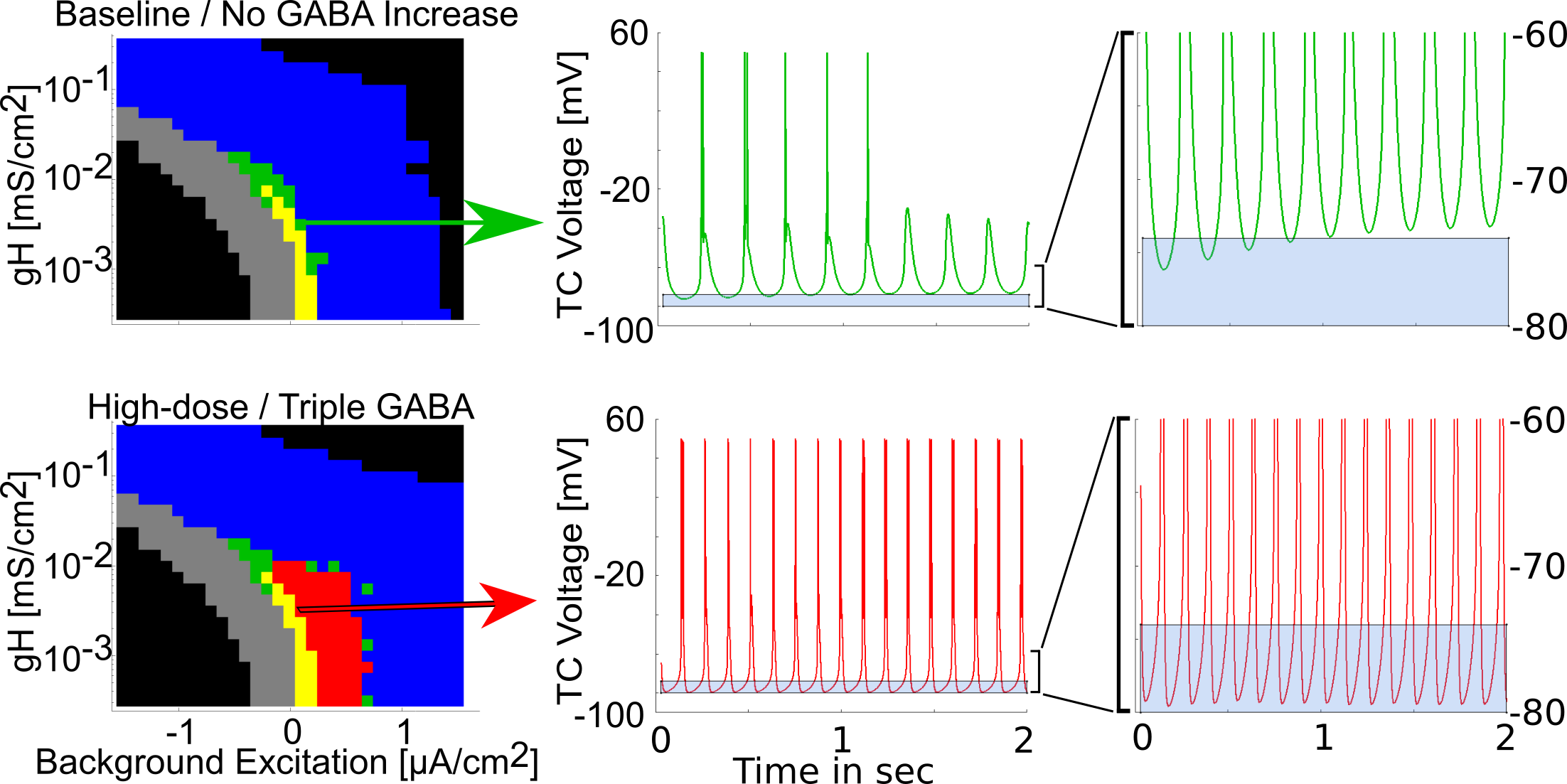 { width=90% }
{ width=90% }
Summary So Far
- Propofol thalamic alpha takes advantage of thalamic spindle dynamics (e.g.
$T_{window}$)
- Enhanced inhibition enables more spiking/oscillating due to T-current and
H-current interplay
Overview So Far
- Increase of $GABA_A$ and decrease of TC cell H-current are required for
thalamic Alpha oscillations
- Thalamic Alpha oscillations are sustained spindles
- Interaction between thalamic Alpha and Slow Wave Activity can produce
propofol phase-amplitude coupling regimes
Alpha-SWO Coupling
Slow Wave Oscillations
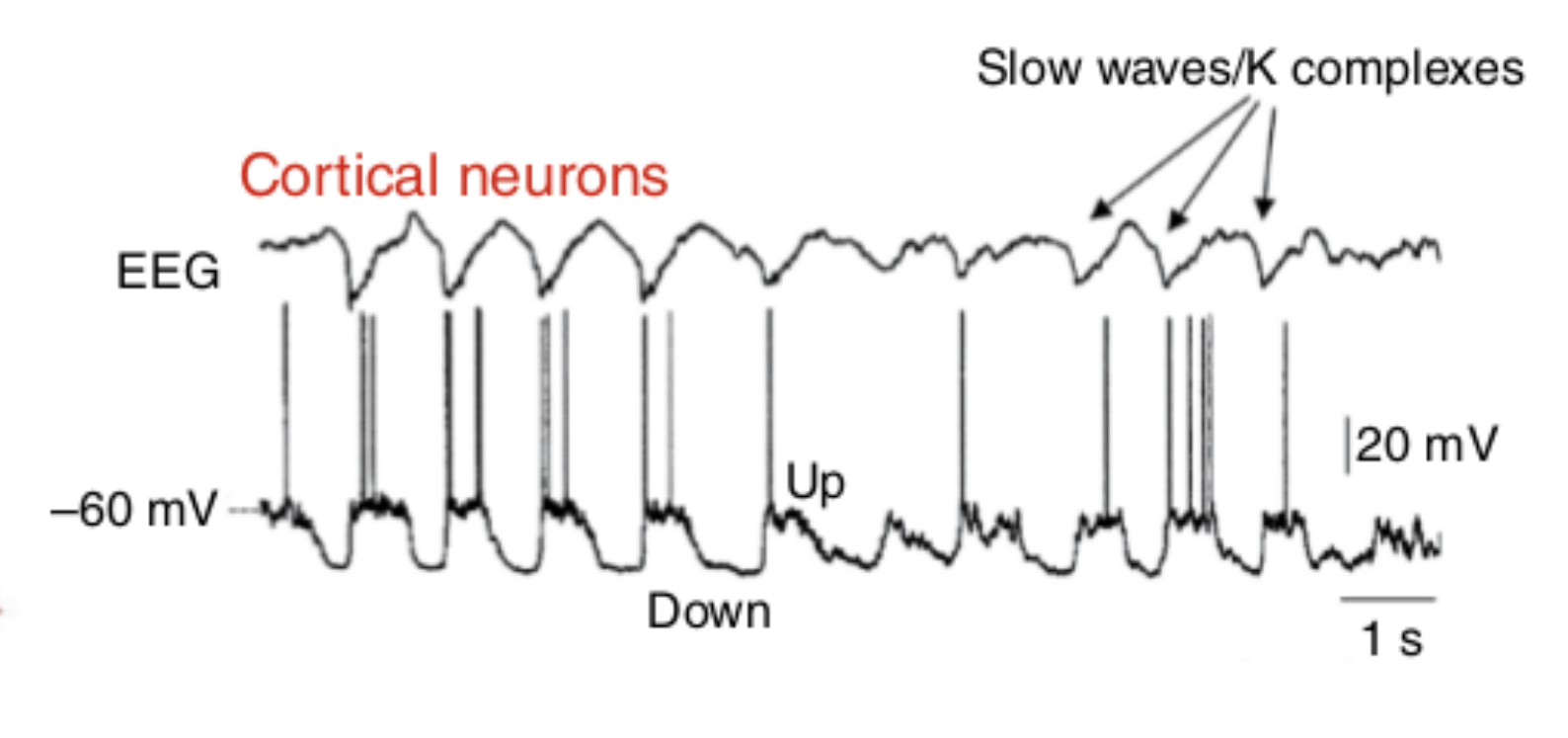 {
width=100% }
{
width=100% }
Phase-amplitude Coupling Switches
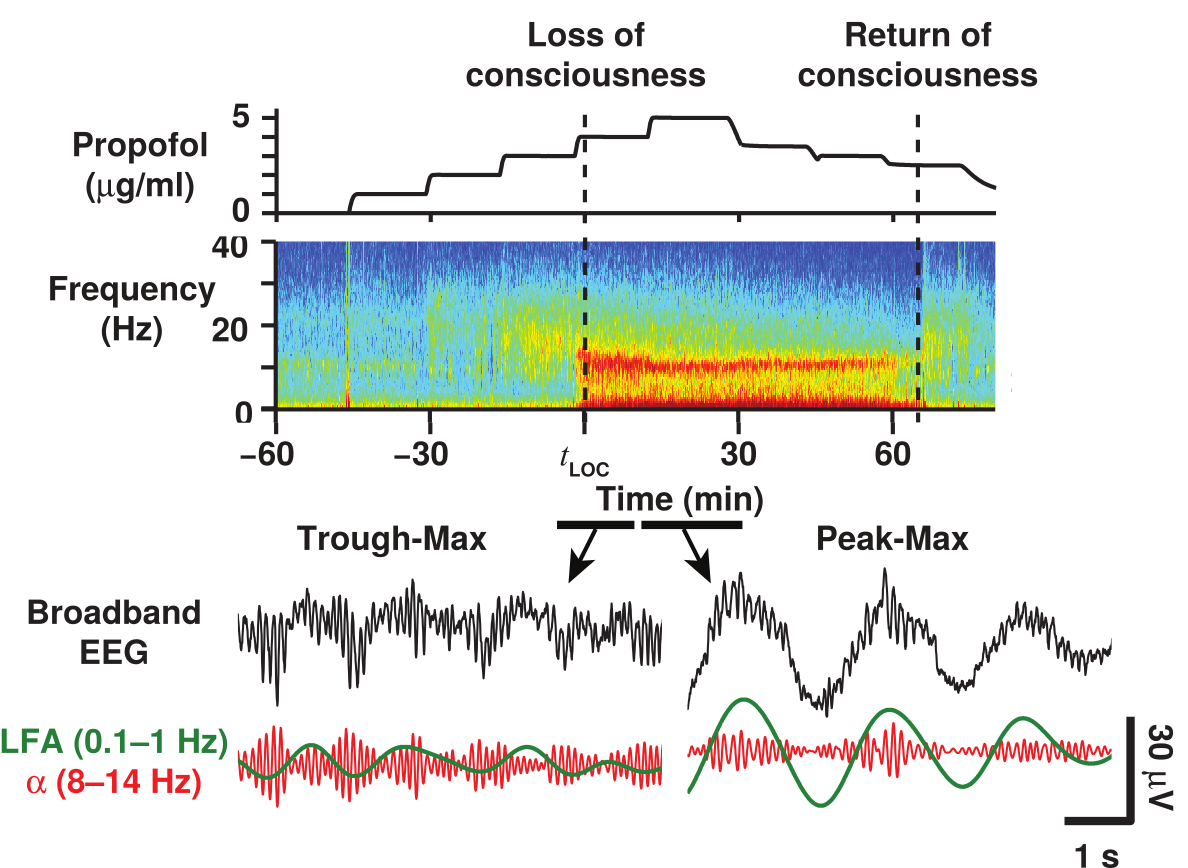 { width=70%
}
{ width=70%
}
Our Full Model Network
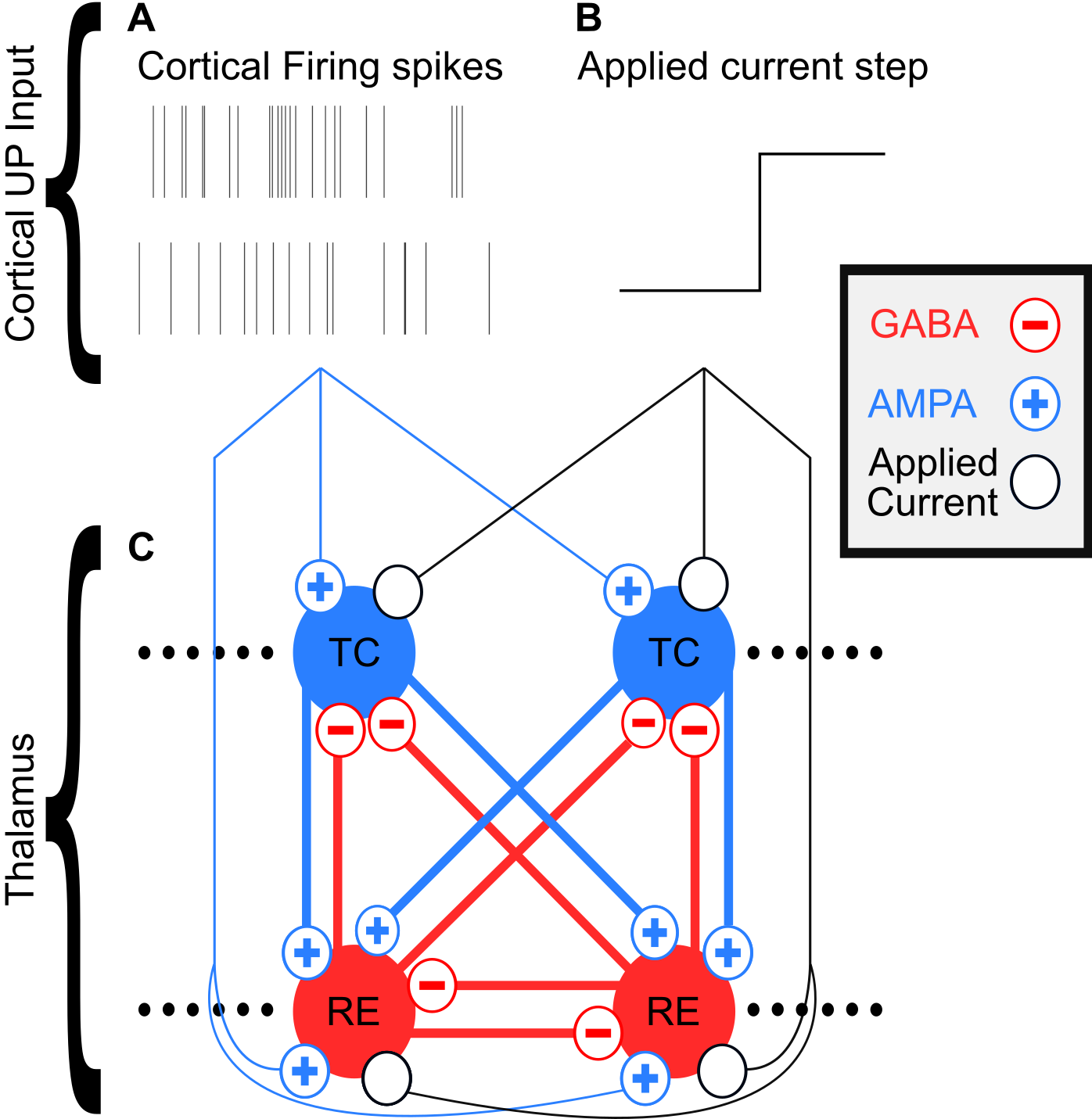 {
width=60% }
{
width=60% }
Simulating UP vs DOWN states
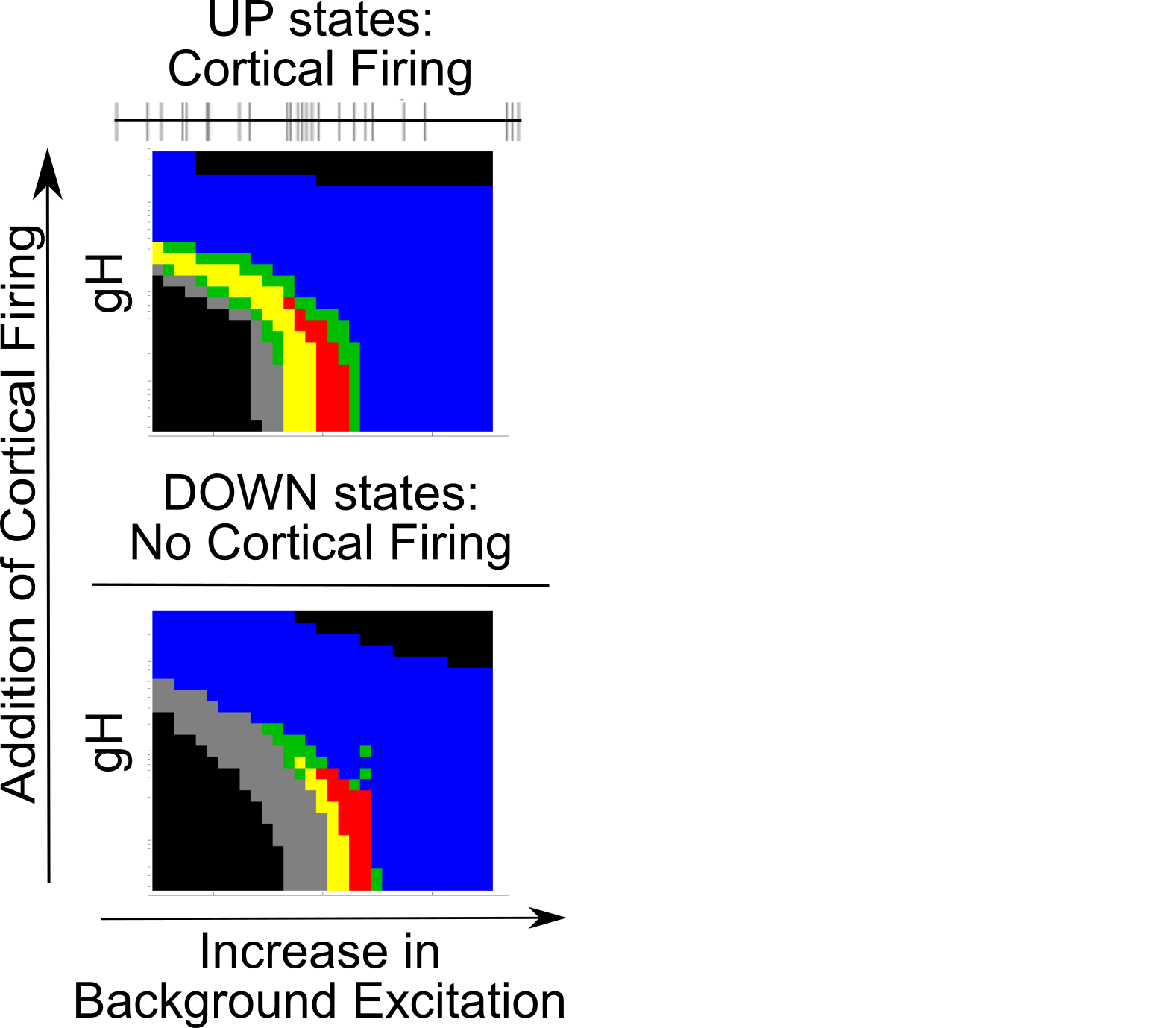 {
width=70% }
{
width=70% }
Simulating UP vs DOWN states
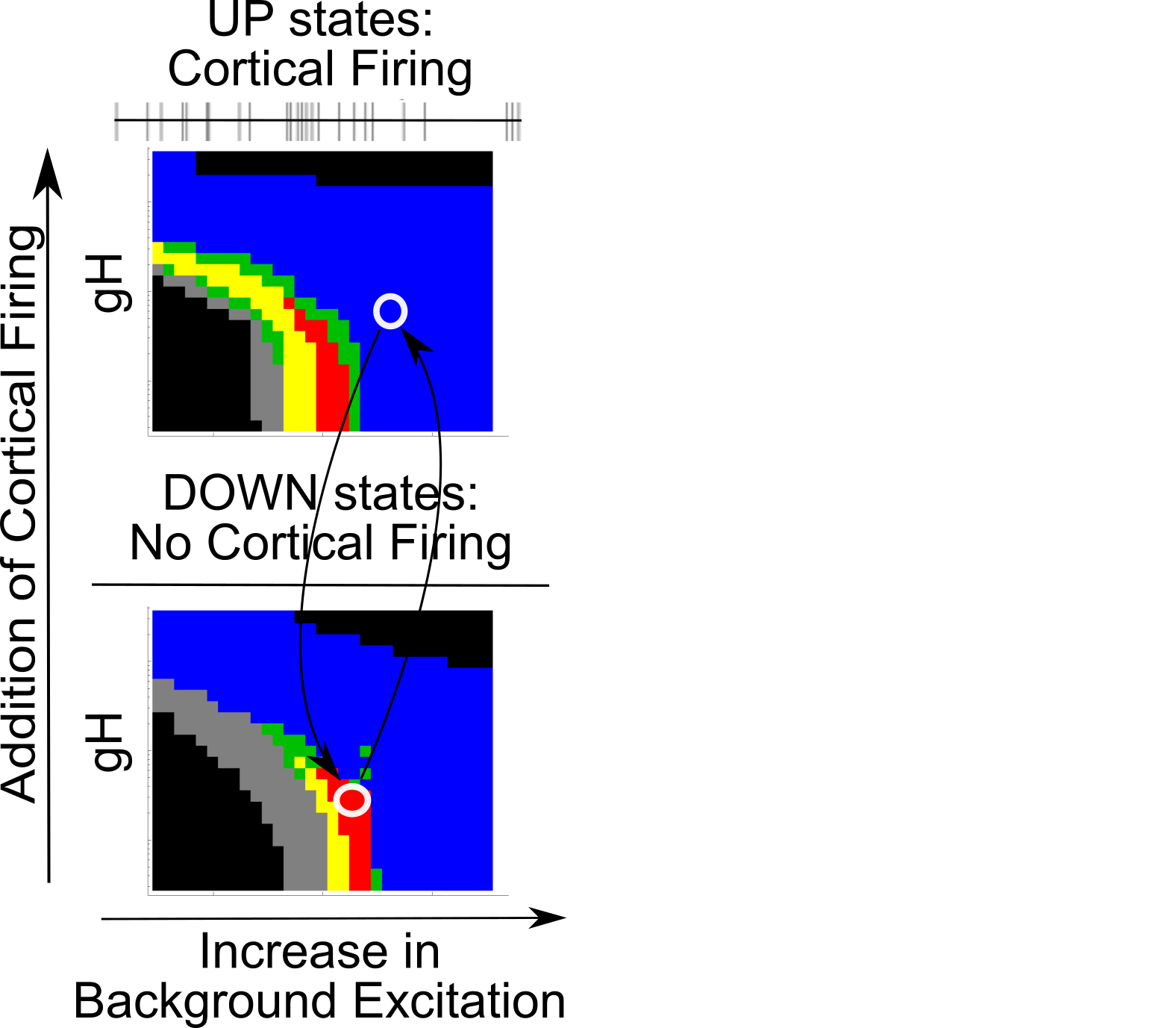 {
width=70% }
{
width=70% }
Trough-max thalamic alpha
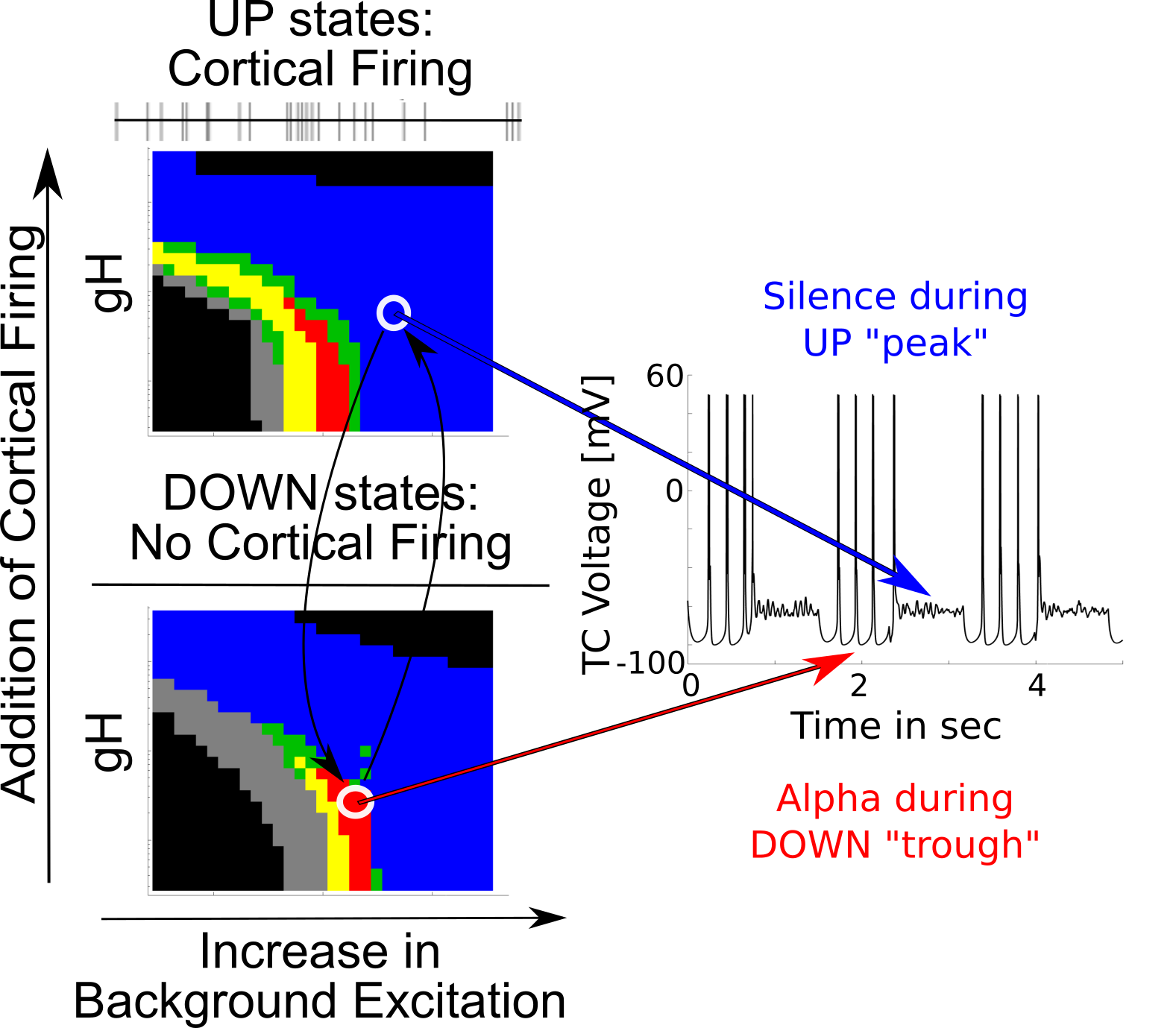 {
width=70% }
{
width=70% }
Trough-max comparison
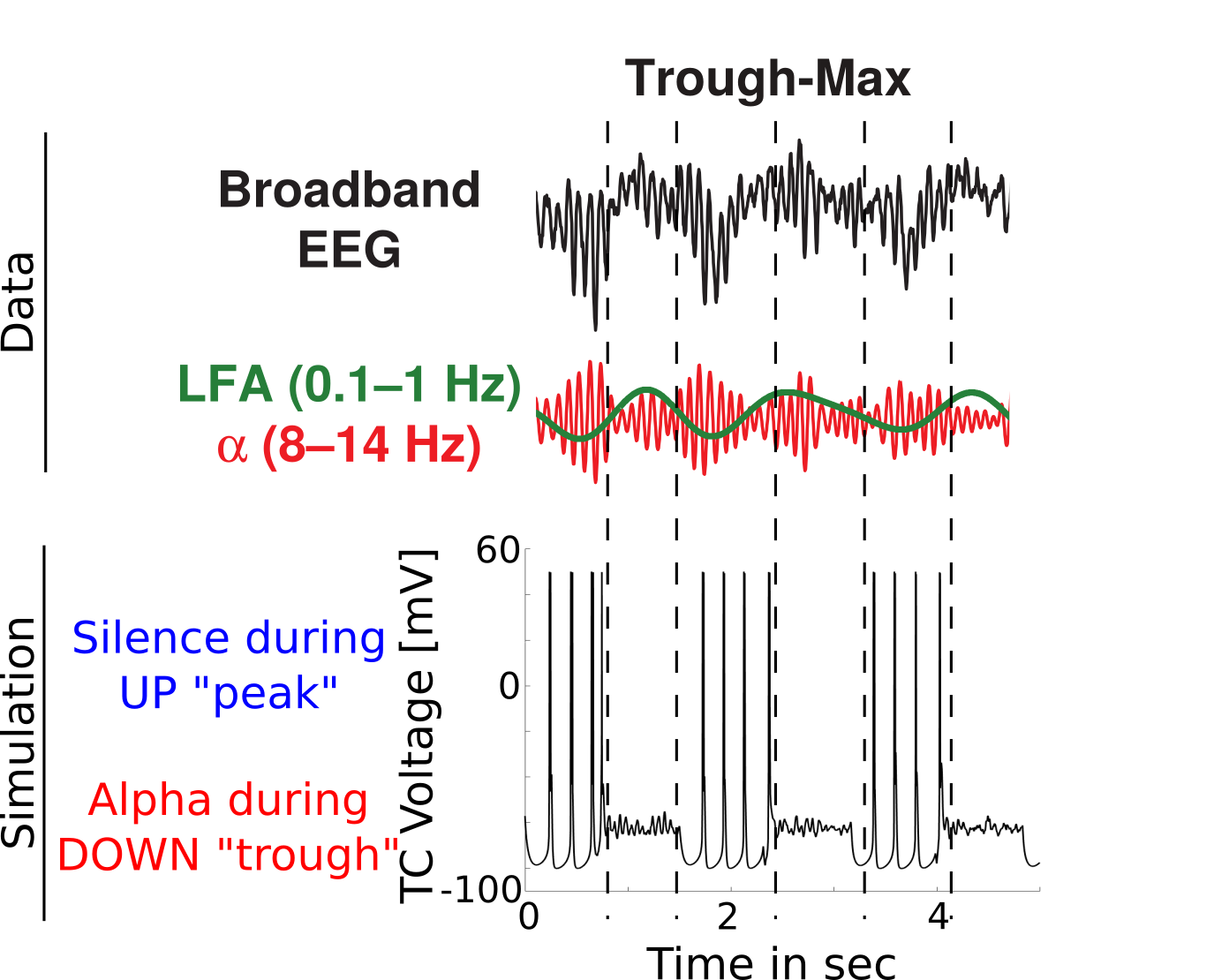 {
width=70% }
{
width=70% }
Peak-max thalamic alpha
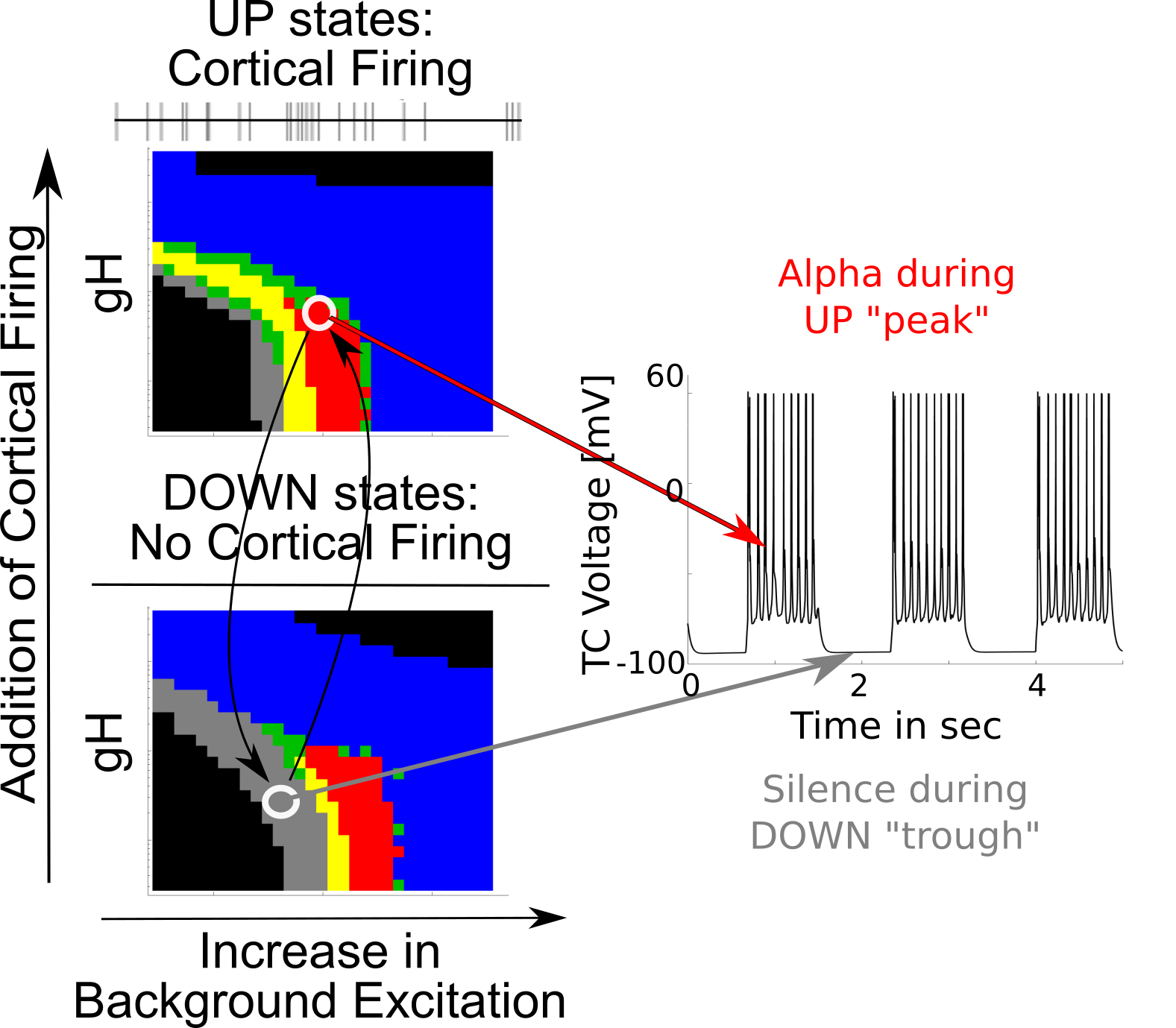 { width=70% }
{ width=70% }
Peak-max comparison
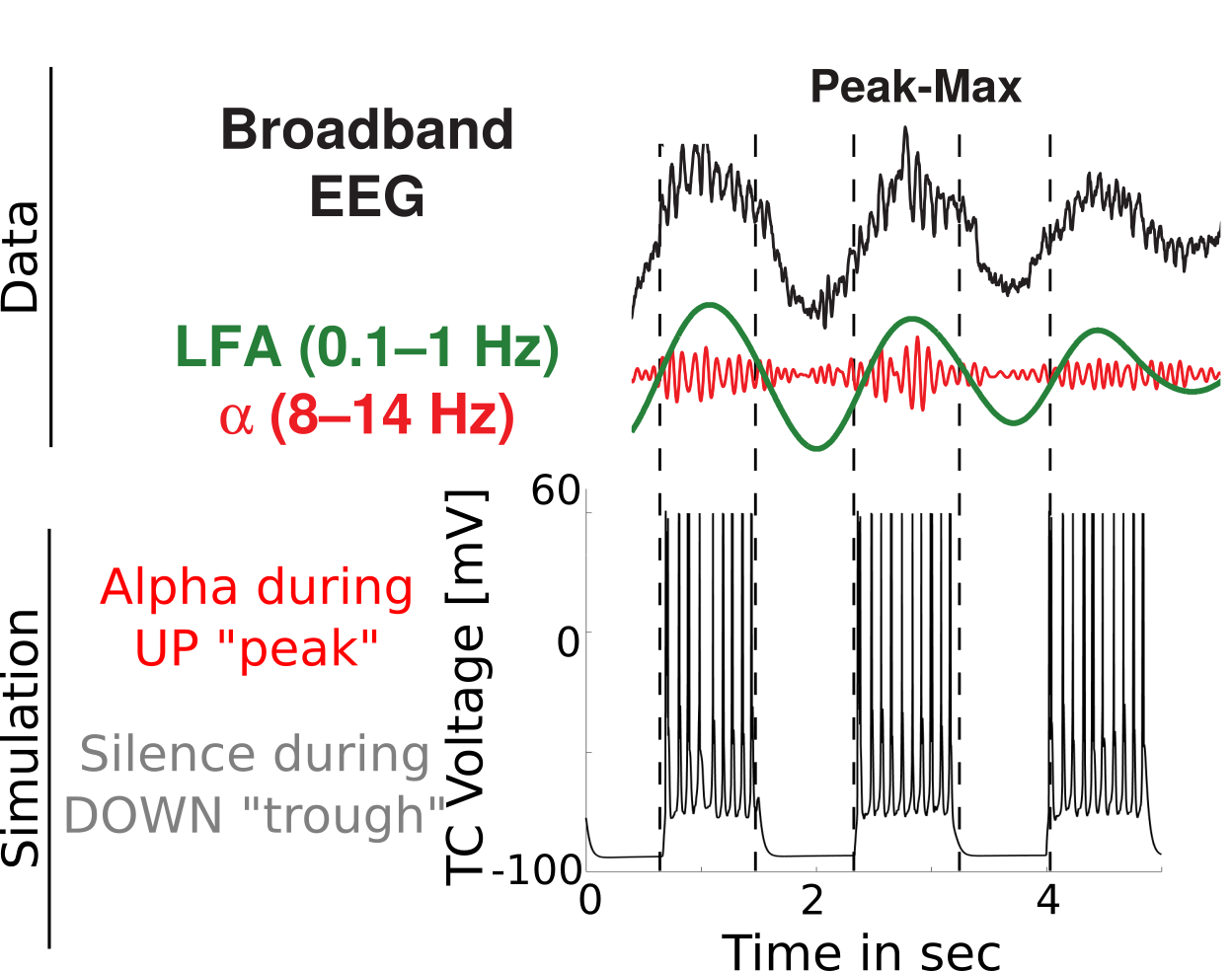 {
width=70% }
{
width=70% }
Coupling Summary So Far
- Given SWO UP/DOWN transitions coming from cortex to thalamus,
- “trough-max” Alpha can be generated during DOWNs by the thalamus
- “peak-max” Alpha can be generated during UPs by the thalamus
- Overall thalamic hyperpolarization is the critical factor for switching
the thalamus between trough-max and peak-max
Conclusions
Conclusions 1
- Propofol sustained alpha may come from its $GABA_A$ increase and
H-current decrease in the thalamus.
- This propofol alpha is dependent on the spindling dynamics of the
thalamus.
Conclusions 2
- During “trough-max” propofol coupling, the thalamus may cause
the sustained Alpha in the DOWN/trough phase. Similarly, in “peak-max”
coupling, the thalamus may cause the sustained Alpha seen during
the UP/peak phase.
- Increased hyperpolarization of the thalamus is sufficient to switch
from trough-max thalamic firing to peak-max thalamic firing, and vice versa.
Implications
- Propofol alpha may arise from the thalamus.
- Hyperpolarization level of the thalamus may determine which coupling regime
is present (trough-max or peak-max), and may be controlled by specific
brainstem nuclei.
- Since propofol alpha is not present during trough-max UP states, there may
still be corticothalamic communication during trough-max.
Acknowledgements
- Kopell Lab @ BU: Nancy Kopell, Michelle McCarthy, Jason Sherfey, Erik
Roberts, alums Shane Lee, ShiNung Ching, Sujith Vijayan
- CRC community
- BU Graduate Program for Neuroscience, especially Shelley Russek and Sandi
Grasso
- Anesthesia research @ MIT: Emery Brown lab, Patrick Purdon lab, Christa van
Dort lab, Ken Solt lab
- NIH, NSF, and HHS for funding including training
Simulation Code
Our lab uses and develops the DynaSim
Simulator originally created by Jason
Sherfey. All the code necessary to run these simulations is available on
GitHub here!
 { width=100% }
{ width=100% }
Appendix
Detail: $T_{window}$ is critical
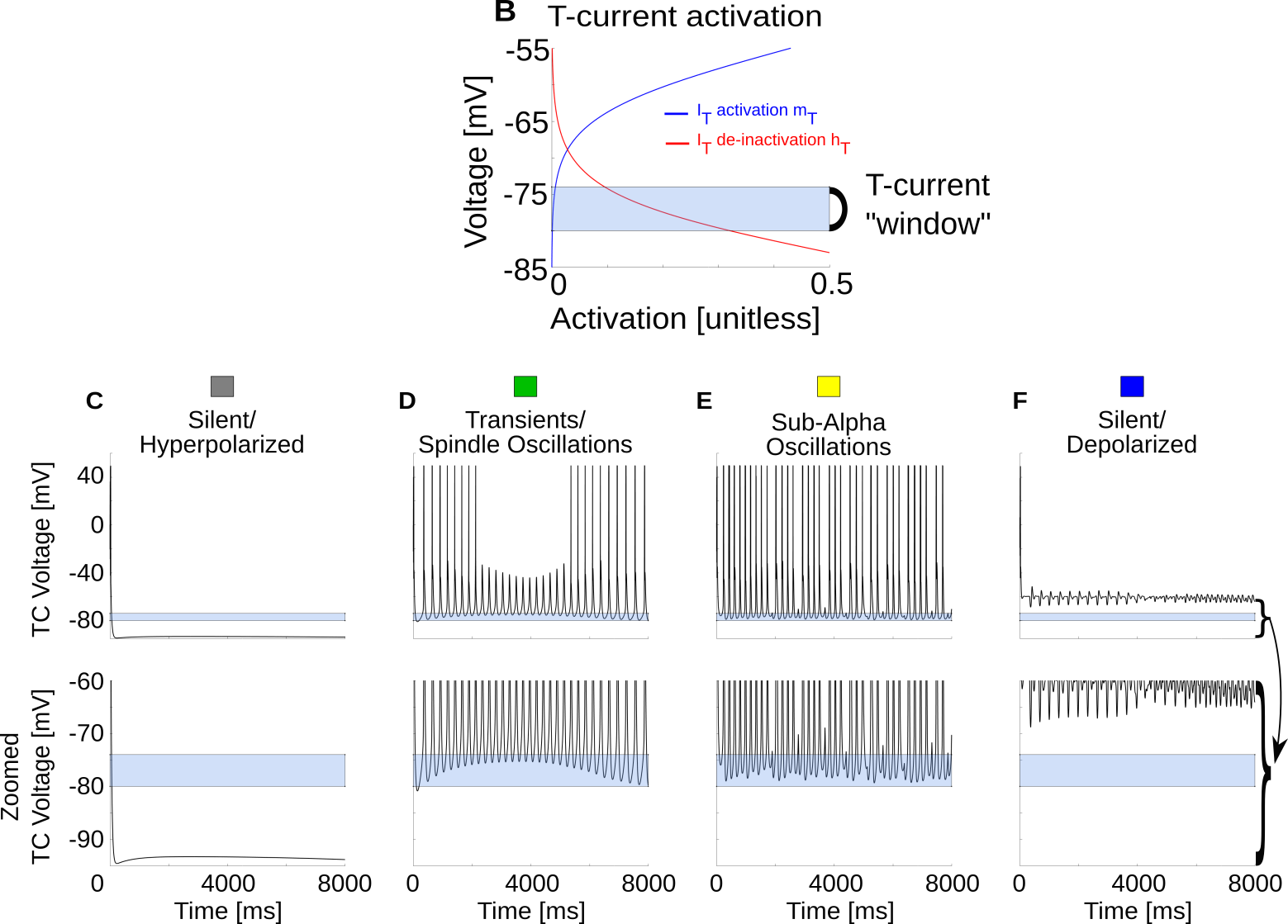 { width=85% }
{ width=85% }
Detail: Propofol Alpha mechanism
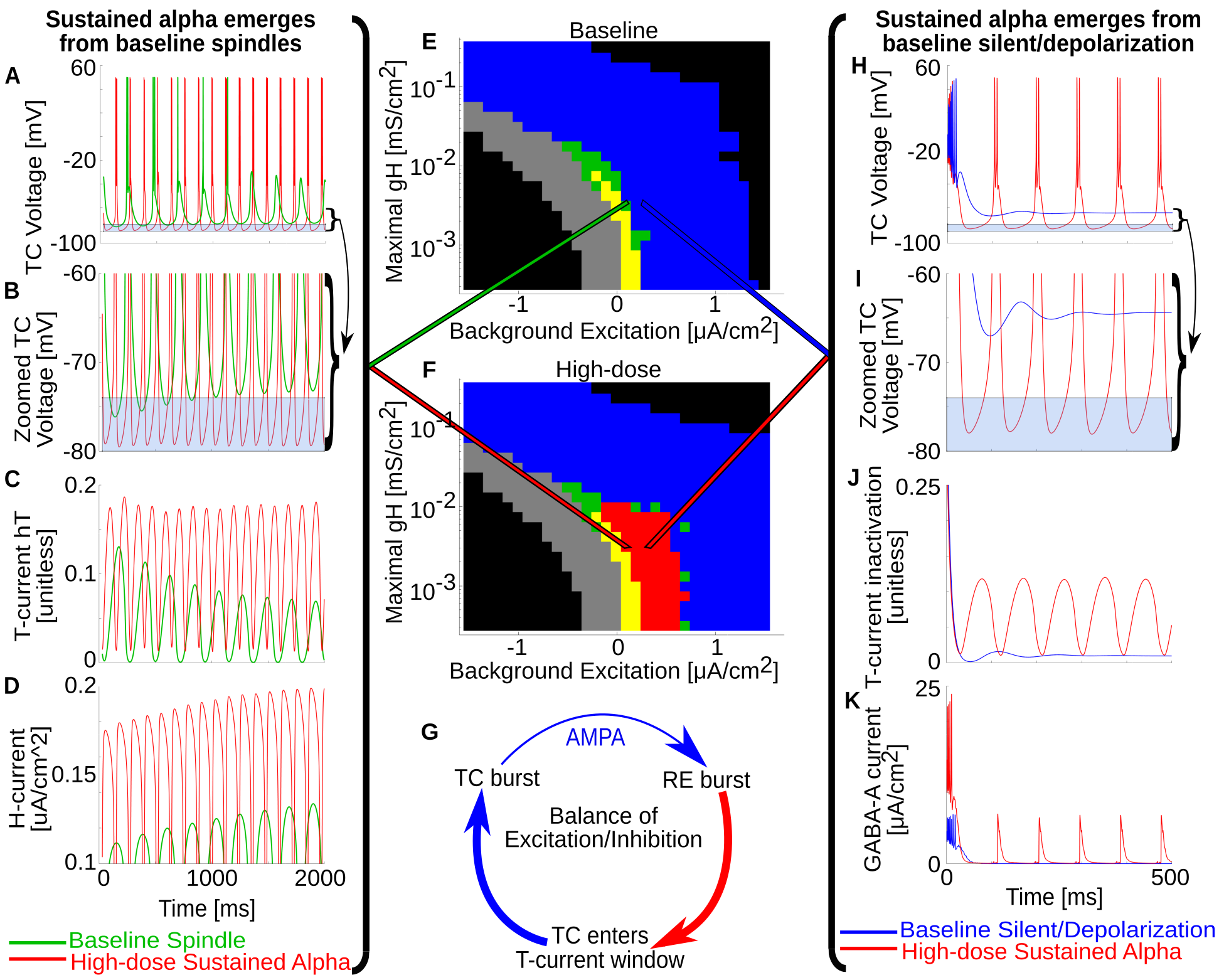 { width=75% }
{ width=75% }
References
CSS
##
 { width=100%
}
{ width=100%
} { width=100%
}
{ width=100%
} {
width=100% }
{
width=100% } { width=80% }
{ width=80% } { width=75% }
{ width=75% } { width=55% }
{ width=55% } {
width=65% }
{
width=65% } { width=90% }
{ width=90% } {
width=100% }
{
width=100% } { width=70%
}
{ width=70%
} {
width=60% }
{
width=60% } {
width=70% }
{
width=70% } {
width=70% }
{
width=70% } {
width=70% }
{
width=70% } {
width=70% }
{
width=70% } { width=70% }
{ width=70% } {
width=70% }
{
width=70% } { width=100% }
{ width=100% } { width=85% }
{ width=85% } { width=75% }
{ width=75% }